Phenolic Acids & Alcohols Standard Mixture - 1
TABLE OF COMPOSITION
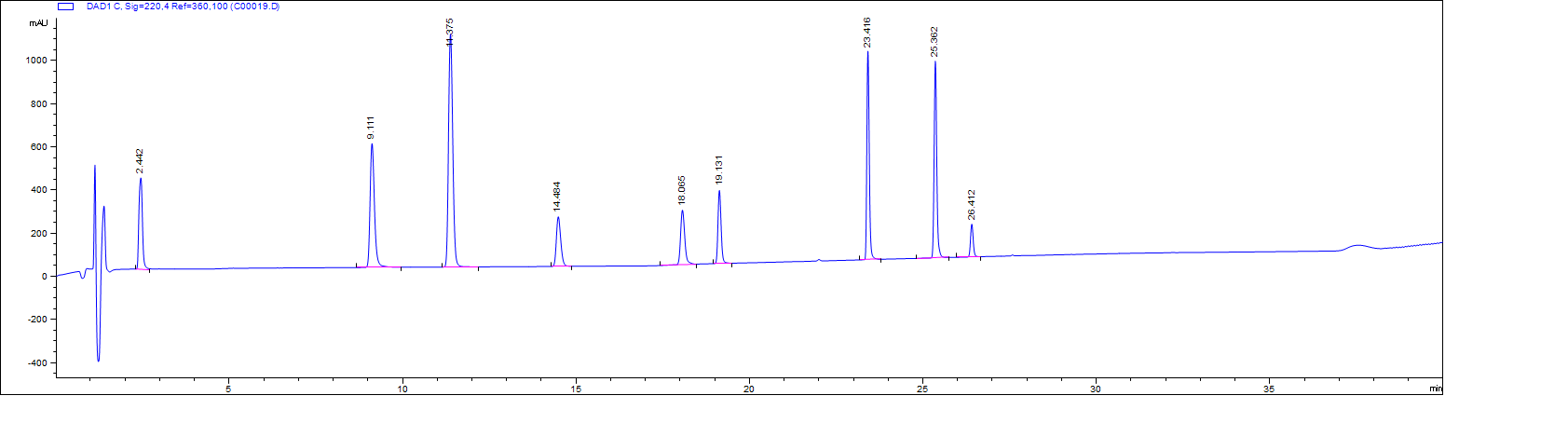
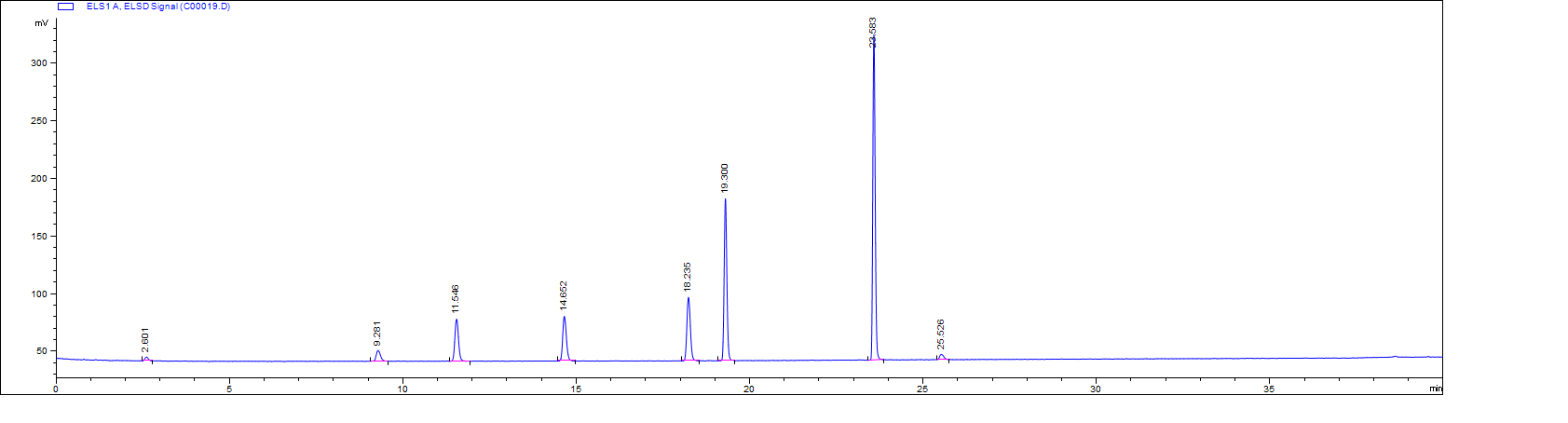
ANALYSIS RESULTS
Agilent HPLC 1290 Series
UV Detector: Agilent 1260 Infinity II Diode Array
ELSD Detector: 1260 Infinity II Evaporative Light Scattering Detector.
MSD: Agilent 6460C QQQ
InfinityLab Poroshell 120 EC-C18, 2.1 x 150 mm, 1.9 µm, narrow bore LC column
| A: 0.1% Formic acid in H2O |
B: 0.1% Formic acid in Acetonitrile | ||
| Time (min) | A (%) | B (%) | Flow (mL/min) |
| 0 | 95 | 5 | 0.3 |
| 2 | 95 | 5 | 0.3 |
| 15 | 88 | 12 | 0.3 |
| 35 | 45 | 55 | 0.3 |
| 36 | 0 | 100 | 0.3 |
| 38 | 0 | 100 | 0.3 |
| 39 | 95 | 5 | 0.3 |
| 40 | 95 | 5 | 0.3 |
All signals in the above chromatogram are recorded at 220nM.
A peak at 13.7 belongs to 0.5mM TBHQ (tert-butylhydroquinone, CAS# 1948-33-0) which is used as an internal standard. The TBHQ in all solutions comes from the same stock mix and is comparable ampule to ampule.
Please refer to the online version of the Certificate of Analysis by scanning the QR code on the product label. Click on the name of the corresponding compound in the Sec 3. Table of composition to view the compound specification.
STABILITY
A shelf life of at least 2 years has been established for this product.
Your product expires no eariler than Feb 2025
No data is available at this moment. For the latest stability results, please visit our website (www.metasci.ca)
Additional Methods
Agilent QQQ 6460C - ESI
InfinityLab Poroshell 120 EC-C18, 2.1 x 150 mm, 1.9 µm, narrow bore LC column
| Name | Polarity | Exact Mass | CAS# | Fragmentor | Precursor Ion | Product Ion | CE | CAV | Abundance | |
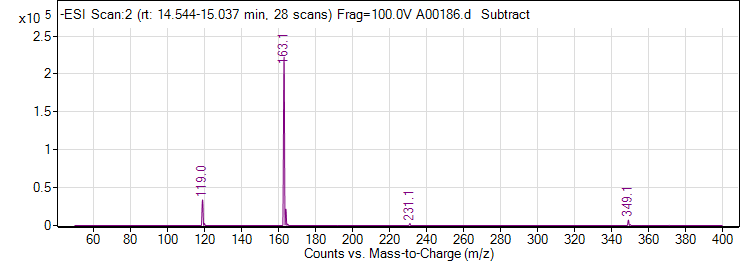
| 1 | p-Coumaric acid | Negative | 164.0473 | 7400-08-0 | 104 | 163 | 119.1 | 10 | 4 | 46359 |
| 1 | p-Coumaric acid | Negative | 164.0473 | 7400-08-0 | 104 | 163 | 93.1 | 38 | 4 | 2651 |
| 1 | p-Coumaric acid | Negative | 164.0473 | 7400-08-0 | 104 | 163 | 117.1 | 34 | 4 | 1238 |
| 1 | p-Coumaric acid | Positive | 164.0473 | 7400-08-0 | 100 | 165.1 | 91.1 | 26 | 4 | 7725 |
| 1 | p-Coumaric acid | Positive | 164.0473 | 7400-08-0 | 100 | 165.1 | 147.1 | 10 | 4 | 13449 |
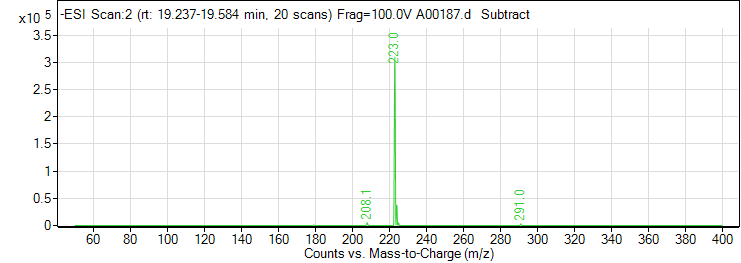
| 2 | Sinapic acid | Negative | 224.0685 | 530-59-6 | 108 | 223.1 | 93.1 | 38 | 4 | 4480 |
| 2 | Sinapic acid | Negative | 224.0685 | 530-59-6 | 108 | 223.1 | 208.1 | 10 | 4 | 8377 |
| 2 | Sinapic acid | Negative | 224.0685 | 530-59-6 | 108 | 223.1 | 121 | 30 | 4 | 5166 |
| 2 | Sinapic acid | Positive | 224.0685 | 530-59-6 | 88 | 225.1 | 91.2 | 30 | 4 | 8325 |
| 2 | Sinapic acid | Positive | 224.0685 | 530-59-6 | 88 | 225.1 | 207.1 | 2 | 4 | 28368 |
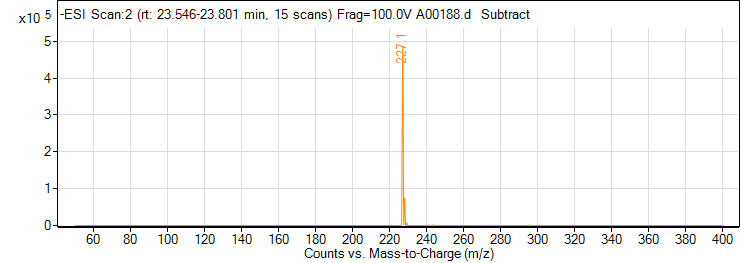
| 3 | Resveratrol | Negative | 228.0786 | 501-36-0 | 141 | 227.1 | 143.1 | 26 | 4 | 5649 |
| 3 | Resveratrol | Negative | 228.0786 | 501-36-0 | 141 | 227.1 | 185.1 | 18 | 4 | 4331 |
| 3 | Resveratrol | Negative | 228.0786 | 501-36-0 | 141 | 227.1 | 41.2 | 34 | 4 | 936 |
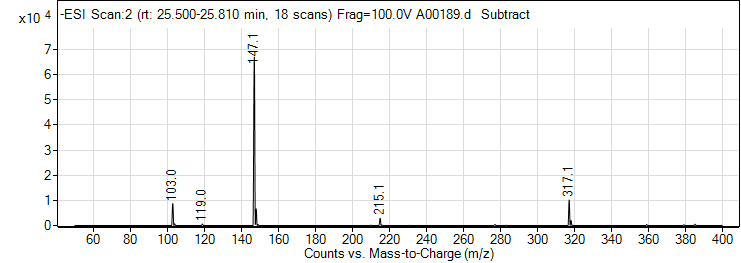
| 4 | trans-Cinnamic acid | Negative | 148.0524 | 140-10-3 | 84 | 147 | 96.6 | 10 | 4 | 52 |
| 4 | trans-Cinnamic acid | Negative | 148.0524 | 140-10-3 | 82 | 147 | 103.1 | 10 | 4 | 761 |
| 4 | trans-Cinnamic acid | Positive | 148.0524 | 140-10-3 | 42 | 149.1 | 85 | 10 | 4 | 7807 |
| 4 | trans-Cinnamic acid | Positive | 148.0524 | 140-10-3 | 42 | 149.1 | 77.2 | 38 | 4 | 3290 |
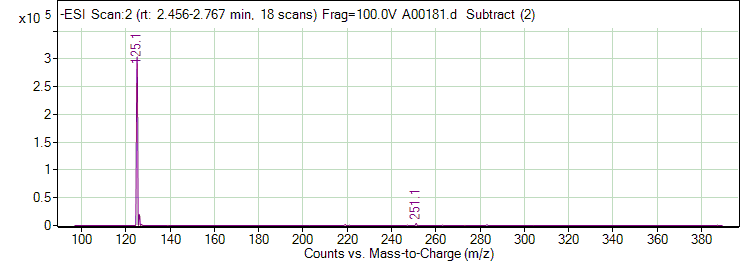
| 5 | Pyrogallol | Negative | 126.0317 | 87-66-1 | 128 | 125 | 79.2 | 18 | 4 | 433 |
| 5 | Pyrogallol | Negative | 126.0317 | 87-66-1 | 128 | 125 | 51.2 | 30 | 4 | 250 |
| 5 | Pyrogallol | Positive | 126.0317 | 87-66-1 | 42 | 149 | 85.1 | 10 | 4 | 8003 |
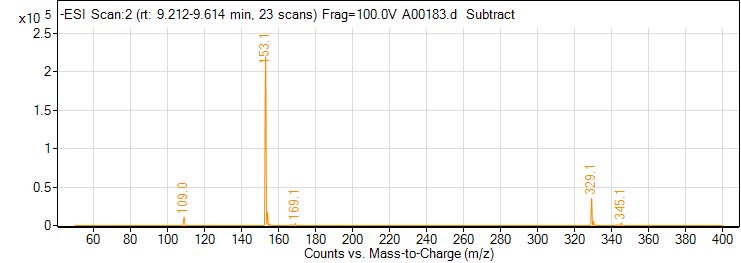
| 6 | 2,3-Dihydroxybenzoic acid | Negative | 154.0266 | 303-38-8 | 94 | 153 | 109.1 | 14 | 4 | 8946 |
| 6 | 2,3-Dihydroxybenzoic acid | Negative | 154.0266 | 303-38-8 | 94 | 153 | 108 | 26 | 4 | 2839 |
| 6 | 2,3-Dihydroxybenzoic acid | Positive | 154.0266 | 303-38-8 | 76 | 155 | 107.2 | 22 | 4 | 115 |
| 6 | 2,3-Dihydroxybenzoic acid | Positive | 154.0266 | 303-38-8 | 76 | 155 | 63.1 | 34 | 4 | 345 |
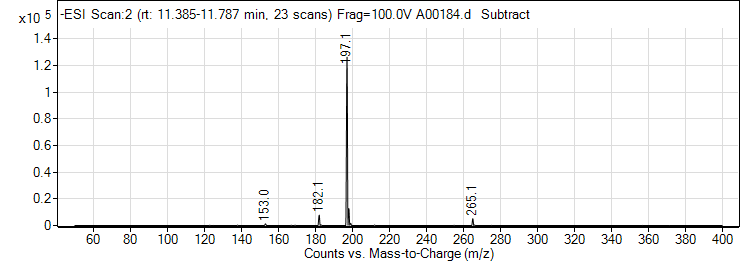
| 7 | Syringic acid | Negative | 198.0528 | 530-57-4 | 106 | 197 | 129.3 | 10 | 4 | 66 |
| 7 | Syringic acid | Negative | 198.0528 | 530-57-4 | 108 | 197 | 182.1 | 10 | 4 | 2999 |
| 7 | Syringic acid | Negative | 198.0528 | 530-57-4 | 108 | 197 | 123 | 22 | 4 | 2452 |
| 7 | Syringic acid | Negative | 198.0528 | 530-57-4 | 108 | 197 | 95.1 | 34 | 4 | 1152 |
| 7 | Syringic acid | Positive | 198.0528 | 530-57-4 | 42 | 199.1 | 85 | 18 | 4 | 3044 |
| 7 | Syringic acid | Positive | 198.0528 | 530-57-4 | 42 | 199.1 | 117 | 10 | 4 | 1339 |
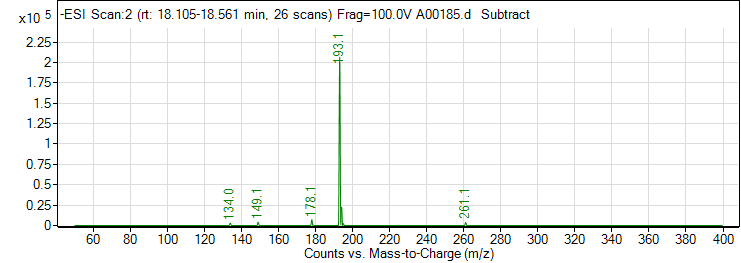
| 8 | Ferulic acid | Negative | 194.0579 | 1135-24-6 | 98 | 193.1 | 178.1 | 10 | 4 | 6900 |
| 8 | Ferulic acid | Negative | 194.0579 | 1135-24-6 | 98 | 193.1 | 149.1 | 10 | 4 | 3196 |
| 8 | Ferulic acid | Negative | 194.0579 | 1135-24-6 | 98 | 193.1 | 134.1 | 14 | 4 | 14447 |
| 8 | Ferulic acid | Positive | 194.0579 | 1135-24-6 | 82 | 195.1 | 85.1 | 18 | 4 | 3558 |
| 8 | Ferulic acid | Positive | 194.0579 | 1135-24-6 | 82 | 195.1 | 177.1 | 10 | 4 | 4037 |
- Solvent system used (refer to the ANALYSIS RESULTS -> Chromatography method):
A: 0.1% Formic acid in H2O
B: 0.1% Formic acid in Acetonitrile
- Experimental: Adding 10-100mM ammonium formate to the solvent system may increase the ionization and the signal of the analytes
- Depending on the ion source and the instrument setup, alcohols generally yield lower signal and fragmentation. In such a case, higher injection volume may improve the signal. When the ionization is too week, the sodium adduct (M+Na) is more prominent.